*Not all course related questions are currently up!
1. Draw the most important Lewis structure for [ IF4 ]+ (assuming it exists) and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom.
(a) What is the electron-group geometry, according to VSEPR theory?
Answer: Trigonal Bipyramidal
(b) What is the molecular geometry?
Answer: See-saw
(c) Is this species polar or nonpolar?
Answer: Polar
2. Draw the most important Lewis structure for [ BrF5 ] and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom.
(a) What is the electron-group geometry, according to VSEPR theory?
Answer:
(b) What is the molecular geometry?
(c) Is this species polar or nonpolar?
3. Draw the most important Lewis structure for [ NO3 ]- and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom.
(a) What is the electron-group geometry, according to VSEPR theory?
(b) What is the molecular geometry?
(c) Is this species polar or nonpolar?
* There are 5 choices. Solution only contains 4 of them
4. Which of the following molecules is/are bent or V-shaped?
HCN
H2O
HN3
CF4
N2O
PF3
H2S
AsH3
SO2
NH3
SO3
BF3
SiF4
C2H2
CS2
COS
O3
5. Predict the geometric shapes of the following.
(a) CSe2
(b) SiF4
(c) AsF5
(d) IF3
(e) SbF5
(f) TeF2
(g) AlF6(3-)
6.

7.
8. What the hybridization schemes for the following species. Choose: (sp, sp2, sp3, sp3d, sp3d2)
(a) PBr6−
(b) CS2
(c) SnBr4
(d) SeO4(2−)
(e) BiCl5
9. In the carbon diselenide, CSe2, identify the number of σ and π bonds, the necessary hybridization scheme and the orbital overlap.
(a) σ bond : _______, π bond :_______
(b) Hybridization scheme of central atom : _______ Choose: (sp, sp2, sp3, sp3d, sp3d2)
(c) Which of the following orbitals on the carbon atom are involved in orbital overlap? Choose all that apply.
s atomic orbitals
p atomic orbitals
sp hybrid orbitals
sp2 hybrid orbitals
sp3 hybrid orbitals
sp3d hybrid orbitals
sp3d2 hybrid orbitals
*Here on Down, I have not created full solutions. But they were covered in the CHEM120 exam.
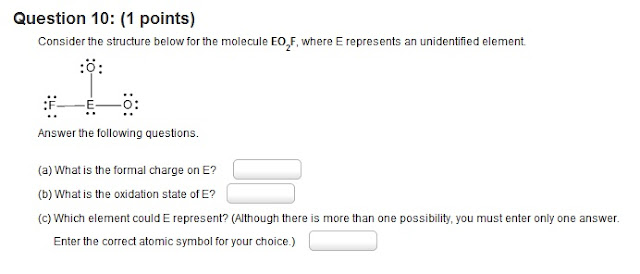
10.
11. Which of the following molecules has/have a permanent dipole moment? You may make one or more selections.
C2H2
O3
AsH3
H2O
HN3
12.Question 12: (1 points) Draw the Lewis structure for the H2CCCH2 molecule and then answer the following questions.
What is the number of sigma bonds?
What is the number of pi bonds?
What is the ideal H-C-C bond angle? (0, 30, 45, 60, 90, 109, 120, 135, or 180.)
What is the ideal C-C-C bond angle? ( 0, 30, 45, 60, 90, 109, 120, 135, or 180.)
13.
14. Which of the following orbitals in N2 has a nodal plane containing both nuclei? You may choose more than one answer.
π*2p
σ2p
σ*2s
σ2s
σ*2p
π2p
15.
(a) According to molecular orbital theory, what is the bond order of B2?
(b) According to molecular orbital theory, what is the bond order of O2(+)?
(c) According to molecular orbital theory, what is the bond order of C2(-)?


No comments:
Post a Comment